Our customers have developed or are in the process of developing lateral flow tests or other point-of-need tests for various applications. These customized OEM solutions can be found in the lab, in the food industry, in veterinary diagnostics, in police cars, and even in outer space!
ESEQuant devices in a wide range of applications
Reference projects
Increasing the efficiency of diagnostic test systems through eHealth solutions
Diagnostic tests are an integral part of many public health interventions. However, delays in the return of test results to physicians are often a problem – especially when timely assessments are critical for effective treatment decisions. eHealth solutions have an important role to play in solving this problem, addressing both market and social needs in high- and low- income settings alike through connected diagnostics.
The Connected Diagnostics Initiative (CDx), initiated by FIND, aims to develop interface tools and messaging standards for instrument manufacturers to harmonize and accelerate availability of connected diagnostics. CDx offers an application programming interface (API) (http://dxapi.org/) that enables the secure transmission of information from diagnostic devices using mobile technology to the CDx management and informatics platform as well as existing applications and databases within health systems to, for example, rapidly inform clinicians, community health workers, patients or national disease surveillance program of test results, issues and trends.
FIND partnered with DIALUNOX to test the ease and speed for a device manufacturer to implement connectivity using an API. Implementation was on the ESEQuant LR3 lateral flow reader, a simple, portable, diagnostic device. The DIALUNOX software team was able to implement connectivity in less than 8 weeks, and the results of MPT64-based TB rapid speciation and line probe assays were successfully transmitted to the CDx platform quickly, securely and reliably using the API.

New hope in the battle against animal epidemics

Outbreaks of animal epidemics can cause enormous economic damage and also place human health at risk. These occur frequently in emerging and developing countries as there are often no suitable early detection and control mechanisms in place.
A program has been developed by the UN Food and Agricultural Organization (FAO) and the International Atomic Energy Agency (IAEA) in 32 developing countries to help combat animal epidemics quicker and more effectively in the future. Fundamental to this program is a convenient device manufactured by DIALUNOX that can be used to identify the causative pathogens.
Quantitative measurement of total IgE in whole blood
Measurement of allergen-specific IgE as well as total serum IgE is used to assess possible allergic reaction, but new fast and easy-to-use screening methods are needed.
A client-developed, gold bead-based colorimetric immunochromatographic test detects IgE directly from whole blood using the ESEQuant Lateral Flow Reader and radio-frequency identification (RFID) reader.
This system reports the total IgE levels in a dynamic range from 30–2000 U/ml. As a safeguard, internal validity points are checked by the reader and only valid results are reported. Lot-specific features are encoded on RFID labels and automatically detected by the lateral flow reader. There is a high correlation between the results from this portable device and a reference laboratory instrument.
Patient-near rapid testing of HPV-related oral cancer using the ESEQuant LR3
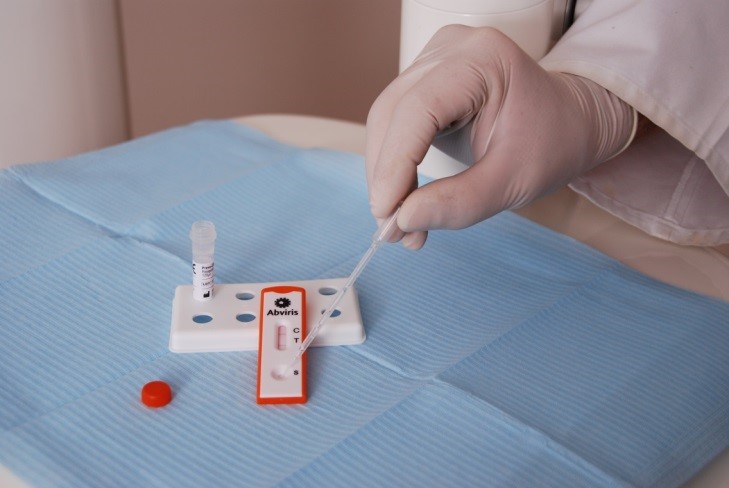
A leading cause of head and neck cancer is the high risk human papilloma virus strain 16 (HPV16). Detection of HPV16-specific antibodies can help to identify this cancer at an early stage and allow tumor resection.
Prevo-Check from Abviris (Huenfelden/Germany) is a lateral flow immunoassay that allows identification of antibodies directed against HPV16 in whole blood samples within 15 minutes using the ESEQuant LR3. This assay provides a convenient way of identifying HPV16-associated cancer at the point-of-need, for example, in the dental practice. This system also allows decentralized monitoring of patients after resection of HPV-induced tumors as these tumors typically have high recurrence rates.
In 2015, this decentralized test system was included as part of the Hamburg City Health Study (HCHS) – the worldwide largest monocentric health study that will run until 2020 and aims to enroll about 45,000 people. As well as collecting medical data, HPV16-specific antibody concentrations in blood will be evaluated in a study cohort. This is in preparation for integrating the test system into a nationwide primary screening program following national S3 guidelines.